
How will the industry evolve during the period between 20? What are the implications of vaccine contract manufacturing projects taking place now and over the next 10 years?.What are the vaccine contract manufacturing projects for these leading companies?.
#Vaccine production usa driver#
What will be the main driver for the overall market from 2023 to 2033?.How will the market shares for each vaccine contract manufacturing submarket develop from 2023 to 2033?.

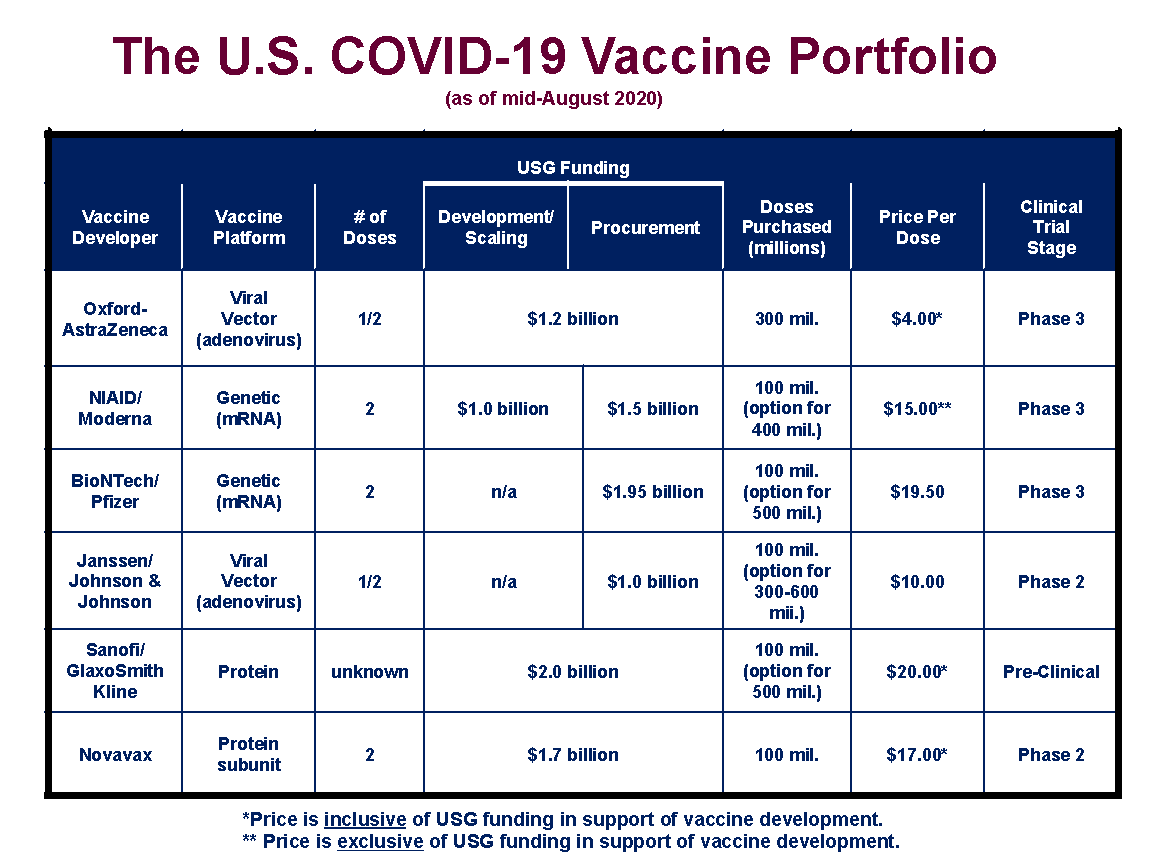
How will each vaccine contract manufacturing submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2033?.What is driving and restraining the vaccine contract manufacturing market?.How is the vaccine contract manufacturing market evolving?.What Questions Should You Ask before Buying a Market Research Report? Limited capacity can result in delays, supply shortages, and increased competition among contract manufacturers for available projects. Contract manufacturers may face challenges in scaling up production to meet the required volumes within tight timelines. The manufacturing capacity for vaccines is limited, and there is often high demand, particularly during pandemics or widespread outbreaks. Negotiating licensing agreements and protecting intellectual property can be challenging and may restrict the participation of certain contract manufacturers in the market. Contract manufacturers may face limitations or restrictions in accessing or using these technologies due to intellectual property rights held by the vaccine developers. Vaccines often involve patented technologies and proprietary manufacturing processes. Adhering to these regulations can be complex and time-consuming, leading to delays in production and increased costs. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The vaccine manufacturing industry is highly regulated, and contract manufacturers need to comply with stringent regulatory requirements imposed by regulatory authorities such as the U.S. Regulations, Intellectual Property and Capacity Challenges to Hamper Industry Growth This cost advantage makes contract manufacturing an attractive option for vaccine developers. Establishing and maintaining large-scale manufacturing facilities can be capital-intensive for vaccine developers, while contract manufacturers can leverage their existing infrastructure, equipment, and expertise to produce vaccines at a lower cost per unit.
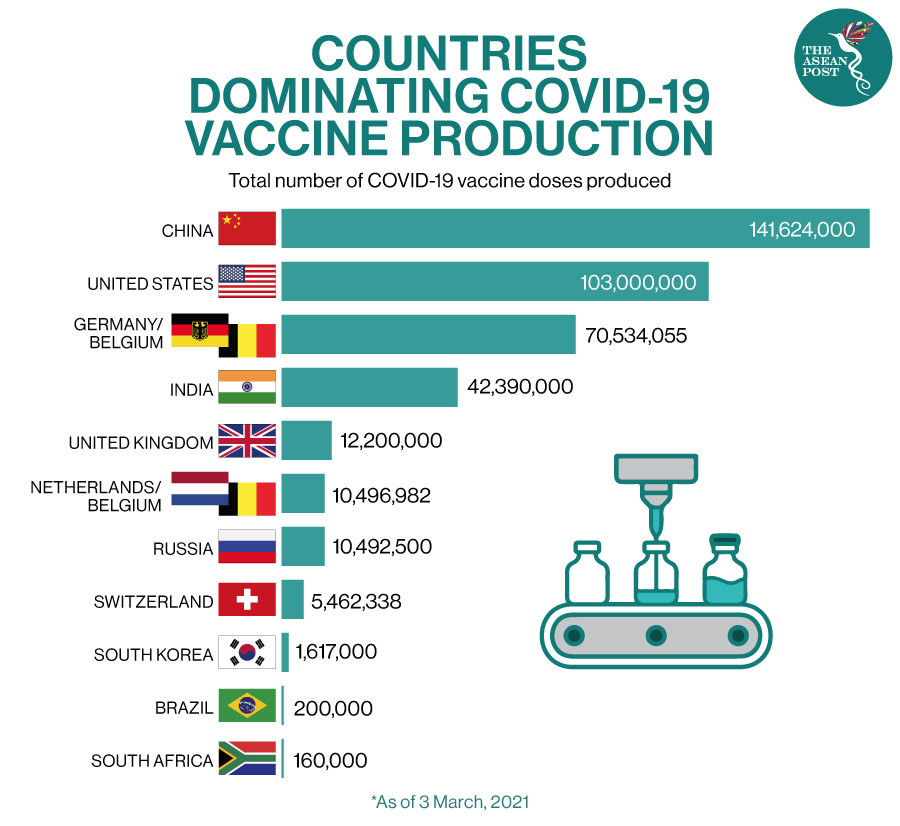
Contract manufacturing can provide cost-effective solutions for vaccine production. As vaccine developers focus on RandD activities, they often seek external partners to handle manufacturing operations, driving the demand for contract manufacturing services. Contract manufacturers play a crucial role in translating these innovations into commercial products. Advances in vaccine research and development have led to the discovery of new vaccines and improved formulations. This increasing demand creates opportunities for contract manufacturers to support vaccine developers in meeting the production needs, especially during pandemics or widespread outbreaks. The global demand for vaccines has been rising due to various factors, including population growth, aging populations, and the emergence of infectious diseases. Increasing Global Demand, Product Innovation and Cost Effectiveness Driving Market Growth It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region. The Vaccine Contract Manufacturing Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. New York, (GLOBE NEWSWIRE) - announces the release of the report "Vaccine Contract Manufacturing Market Report 2023-2033".


 0 kommentar(er)
0 kommentar(er)
